207 rare diseases! Why should we pay so much attention to being "rare"?
originate
Not long ago, the National Health and Wellness Commission and other six departments jointly formulated the "Second Batch of Rare Diseases Catalogue", which newly included 86 rare diseases. Together with the first batch of 121 rare diseases included in 2018, at present, there are 207 rare diseases included in the catalogue in China.
In recent years, the word "rare disease" has appeared more and more frequently in everyone’s field of vision. For example, in the news, the father developed "self-made medicine" for his son’s complex disease, and then in the past two years, the price negotiation of medical insurance was hot, and most of them were related to rare disease drugs.

The "drug god father", who was known on the Internet in 2021, took his son to be injected with gene therapy drugs in June 2022, and was also the first patient with Menkes disease who underwent gene therapy in China | See the watermark for the source.
In the past two years, the medical insurance price negotiations have always been hot headlines, and drugs related to rare diseases are included in the national medical insurance drug list every year | Source: CCTV News
Therefore, many people have the impression that the concept of rare diseases is both familiar and unfamiliar: rare diseases don’t seem so "rare", it seems difficult to treat them, and there don’t seem to be so many experts studying rare diseases …
But these superficial impressions are not enough to describe the whole picture of rare diseases.
What is a rare disease? Really "rare"?
In fact, different countries have different definitions of rare diseases. For example, the United States generally defines it as a disease with fewer than 200,000 people in China (that is, about 1 in 1,500), while the prevalence rate in Europe is less than 1 in 2,000.
According to China’s "Research Report on the Definition of Rare Diseases in China 2021", the definition of rare diseases in China is: "Diseases with neonatal morbidity less than 1/ 10,000, morbidity less than 1/ 10,000, and patients less than 140,000 are classified as rare diseases". The main reason is that China has a large population base. If the definition is too loose, the pressure on drug research and development and medical insurance will be too great.
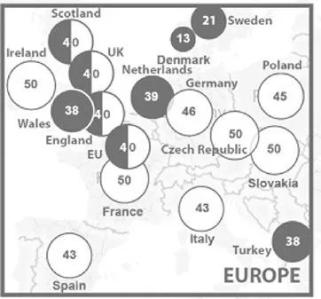
Taking Europe as an example, different countries have different definitions of rare diseases, which often need to be considered according to factors such as population, drug research and development (the unit is every 100,000 people) | Source: Chung C C Y, et al. 2022.
You may be curious: since the prevalence rate is one thousandth or one thousandth, why do you say that it may be too loose to put pressure on the medical system? That’s because the concept of rare diseases is actually a collection of thousands of diseases, or collectively.
According to incomplete statistics, the number of known rare diseases is about 6000-8000. Although the prevalence rate is not all 1/2000, this figure is still very scary-according to the statistical estimation of the incidence rate of 3585 rare diseases, there are about 263-446 million people suffering from various rare diseases all over the world.
This is why, although it is called a rare disease, this group is not "rare" at all.
What does it mean to have a rare disease?
What does it mean to get this rare disease with a probability of one thousandth? Will it become a rare object that is taken care of by everyone like the stars holding the moon?
At least at present, most patients with rare diseases are not so lucky.
First of all, you have to diagnose what disease you are.
But in fact, the diagnosis of rare diseases is very difficult because of limited medical resources and imperfect medical knowledge. About half of the patients with rare diseases in the world still don’t know what disease they have, and the remaining half still face problems such as delayed diagnosis, wrong diagnosis, no treatment plan and unattended care.
What does this mean? If there is a rare patient in Europe, Xiao A, who suddenly has an attack, there is a 25% possibility that Xiao A will not know what disease he has until five years later. In 40% cases, different doctors will give Xiao A different diagnosis and different treatment schemes, and most of these treatment schemes are not helpful to Xiao A because of misdiagnosis.
However, under this wave of operation, Xiao A may have spent a lot of money to cure the disease.
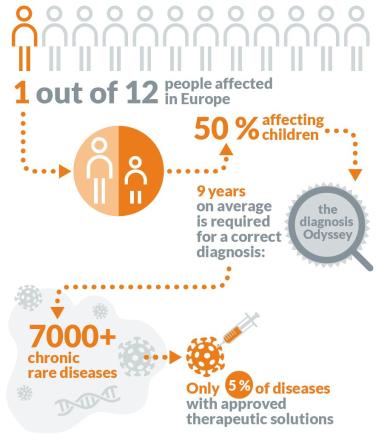
"Layer-by-layer Screening" of Rare Diseases in Europe from Diagnosis to Treatment
In China, however, the shortage of medical resources will only make these ratios higher.
Suppose Xiao A was lucky enough to get an accurate diagnosis in just one year. At this time, the doctor turned over a long list of rare diseases and told Xiao A with regret that there was no medicine for your disease. Because now more than 90% of rare diseases have no good treatment.
This is also the reason why it was mentioned at the beginning that two years ago, the father could only develop drugs to treat his son himself.
As a result, Xiao A will fall into long-term hospitalization or drug treatment, not only can barely maintain his life, but also bear the sky-high medical expenses for life-for example, patients with cystic fibrosis need about 280-1.9 million euros in medical expenses for a lifetime.
Let’s assume that Xiao A is super lucky. Not only is he diagnosed quickly, but he also finds that this rare disease has a very effective orphan drug (rare disease is also called "orphan disease" because the patient is rare, just like "orphan"). As a result, ta may be shocked when he looks at the drug price: it is reported that the average price of rare disease drugs used by each patient is 4.8 times that of other diseases.
You can do a "simple math problem": in 2014, the development of a new drug in China will cost about $1.2 billion, and to maintain the research and development of the whole pharmaceutical industry, it is required that the sales volume should be at least $15 billion. According to the maximum subsidy of 300,000 RMB (about 50,000 US dollars) provided by medical insurance, at least 300,000 patients are needed-and a rare disease is defined as a disease with less than 140,000 patients. Then it requires higher pricing, so that drug researchers and the whole industry can "return to the original".
In other words, patients with rare diseases have to pay more for treatment than other patients.
What does it mean to study rare diseases?
But why are drugs for rare diseases so expensive? Are biomedical companies all black-hearted enterprises? In fact, it is not only patients, but also researchers of rare diseases.
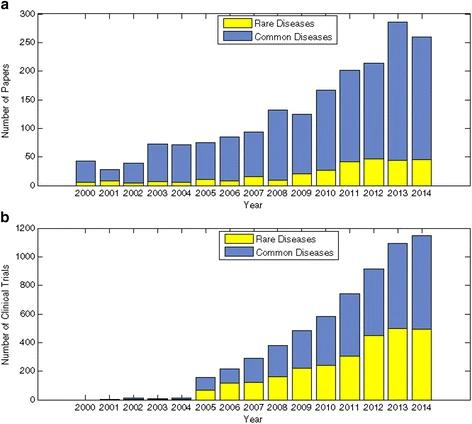
However, the research on rare diseases in China is on the rise, even catching up with common diseases. The above figure shows the number of published research articles, and the following figure shows the number of clinical trials | Source: Yang L, et al.2015.
Suppose researcher Xiao B wants to study a rare disease. The first question Xiao B faces is: Where is my research object?
Although the definition of rare diseases is less than 140,000 people, it sounds like a large number, but in fact only a small number of patients will go to the hospital, and they are scattered in hospitals all over the country.
Not to mention finding a needle in a haystack, it should be as difficult as finding a needle in a swimming pool.
Let’s continue to assume that Xiao B is lucky enough to collect information about dozens of patients with rare diseases in several hospitals and prepare for research. At this time, the question comes again-where does the research funding come from? Rare diseases are mostly genetic diseases, and the study of rare diseases requires gene sequencing, data analysis, animal experiments and so on, which require no less funds than ordinary diseases.
However, from the simple relationship between supply and demand, patients with more common diseases often get more financial support; However, there are more than 5,000 rare diseases with different pathogenesis, so much research funds are available, and what researchers can get is often a drop in the bucket.
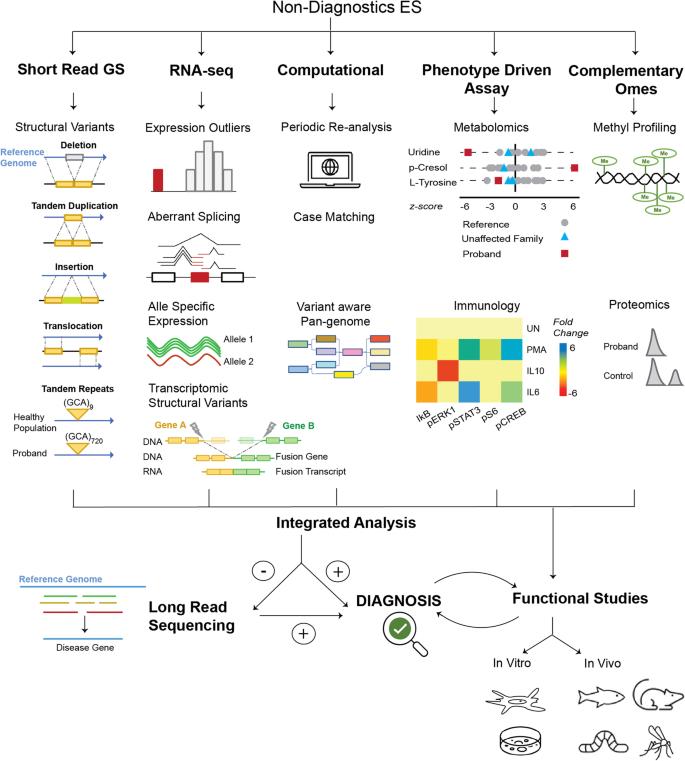
From diagnosis to research and development of rare diseases, every step is money, especially when ordinary sequencing (exon sequencing, ES) is ineffective, it is more complicated for researchers to judge the difficulty and exploration direction of rare diseases | Source: Marwaha S, et al. 2022.
However, assuming that Xiao B is lucky, he just applied to the National Natural Science Foundation of China, which has started to increase funding for research on rare diseases in recent years, and got a lot of research funds. Xiao B intends to do a big job to develop an effective drug for this rare disease and solve this rare disease once and for all!
So Xiao B collected patient information, sequenced to find rare disease targets, and began to screen different drugs;
After discovering hundreds of drugs that may be useful, carry out cell experiments and animal experiments;
Finally, five drugs may be effective, so they went to clinical trials;
After the first phase, the second phase and the third phase of the clinic, I was particularly lucky that a drug was proved to be effective;
Start the long drug approval and enter the fourth phase of clinical practice;
Finally, the drug was put into use.
So more than ten years have passed.
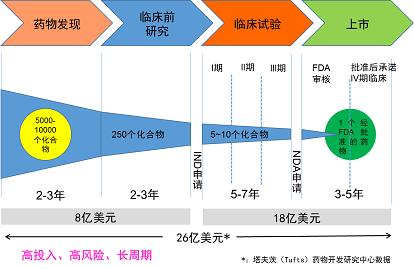
Process of new drug research and development | Source: Shanghai Institute of Pharmacology, China Academy of Sciences
In addition, the results brought by the small sample size of patients are unreliable, the experimental design is difficult, and the treatment is not necessarily extensive and effective, which are puzzling the researchers of rare diseases.
It can be seen that it is a thankless thing for researchers to study rare diseases and develop related new drugs.
We can’t ignore it just because it’s "rare"
However, we can’t ignore hundreds of millions of patients with rare diseases because rare diseases are rare, or because research is "thankless".
For patients, governments all over the world have gradually realized the importance of rare diseases, and have increased investment in relevant medical insurance policies, so that more and more patients can get the best treatment at acceptable prices. There was also a hot search for high-priced drug negotiations in the previous two years.
By the end of this year, more than 50 kinds of 75 kinds of drugs for rare diseases that have been approved for listing in China have been included in the medical insurance drug list.
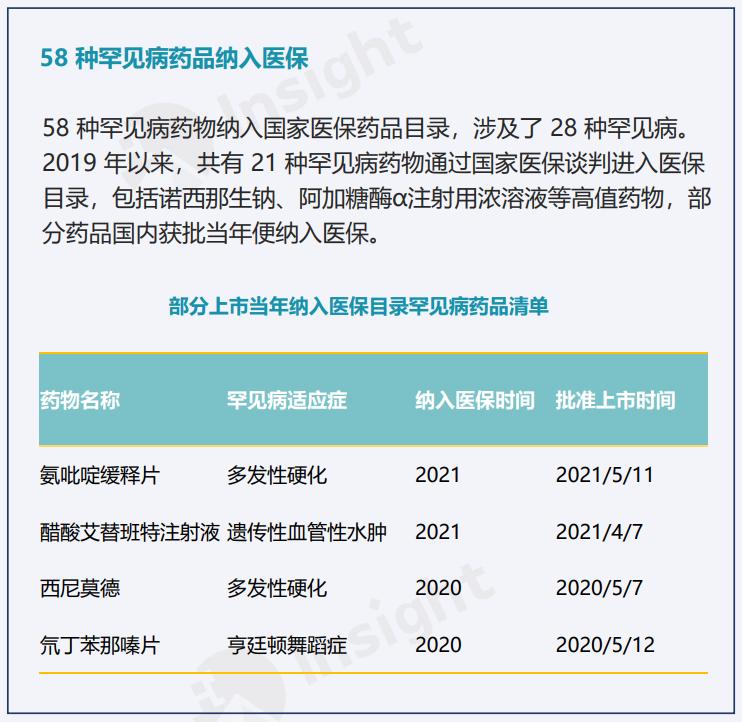
The status quo of rare disease drugs included in medical insurance | Source: Comprehensive report on rare diseases
For the drug research of rare diseases, different countries often support the development of rare diseases drugs from the aspects of research and development funds, drug approval cycle, drug tax relief and so on. In recent years, China has also improved the laws and regulations in this area to better support the research of rare diseases in China.
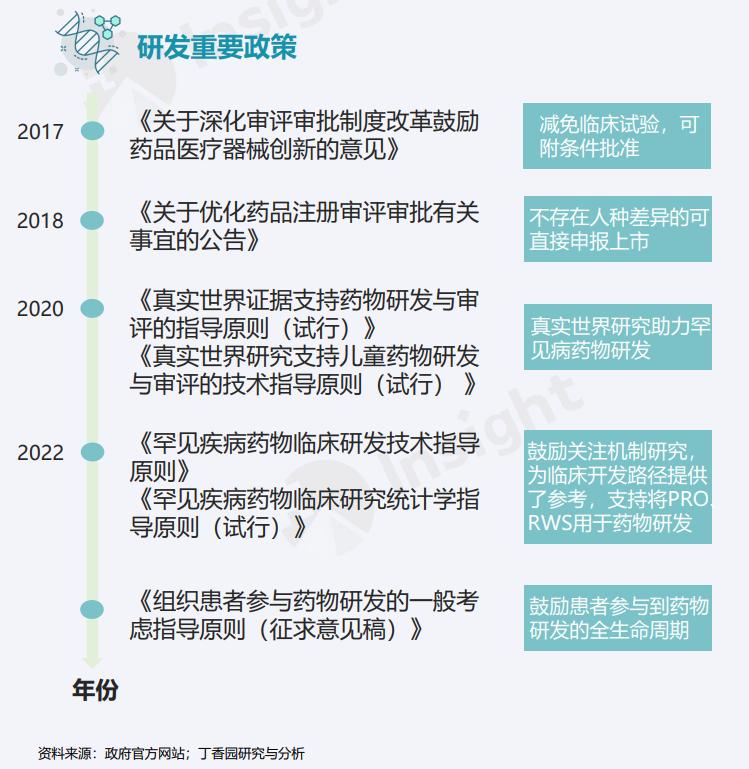
From the end of last century, governments and research institutions in various countries gradually realized that rare diseases are not rare, and they also deserve public attention. From diagnosis to treatment, from research to drugs, there are more and more different supports, but in the face of 300 million patients and thousands of diseases, the progress now is far from enough.
As a trivial biological science popularization person, we also hope to make a contribution in this respect. Now that China has listed 207 rare diseases, we will try to introduce these "orphan diseases" in simple and easy-to-understand language, which you may have never heard of.
I believe that as long as everyone knows something, orphan disease will no longer be lonely.
References:
? Chung C C Y, Hong Kong Genome Project, Chu A T W, et al. Rare disease emerging as a global public health priority[J]. Frontiers in public health, 2022, 10: 1028545.
The Third Multidisciplinary Expert Seminar on the Definition of Rare Diseases/Orphans in China, Research Report on the Definition of Rare Diseases in China 2021.
Comprehensive report on lilac orchard and rare diseases
? Ferreira C R. The burden of rare diseases[J]. American journal of medical genetics Part A, 2019, 179(6): 885-892.
? Yang L, Su C, Lee A M, et al. Focusing on rare diseases in China: are we there yet? [J]. Orphanet journal of rare diseases, 2015, 10(1): 1-2.
? Griggs R C, Batshaw M, Dunkle M, et al. Clinical research for rare disease: opportunities, challenges, and solutions[J]. Molecular genetics and metabolism, 2009, 96(1): 20-26.
Read the original text